Photo-electro catalysis and surface science of metal oxides
Short Bio
Hicham Idriss received his BSc, MSc, PhD and Habilitation (Dr. Science) from the University of Strasbourg (France). Prior to joining KIT, he was Fellow at SABIC Corporate R&D (KAUST), Saudi Arabia (2011-2021), Aberdeen Energy Futures Chair and Professor of Chemistry at the University of Aberdeen and Robert Gordon University (UK) (2008-2011), Associate Professor at the Department of Chemistry, the University of Auckland, New Zealand (1995-2008). His main research area is in catalysis and surface reactions. In the last two decades, he has focused on hydrogen production from renewables.
Hicham is also Professor (Hon.) at the Department of Chemistry, University College London, UK
Emeritus & Honorary staff | Chemistry - UCL – University College London

Research
1. Hydrogen production from water
Fundamental and applied studies of metal/metal oxides single crystals and powder for energy related catalytic processes such as hydrogen from renewable sources and circular systems. In particular metal/metal oxide interface with multijunction solar cells, as an integrated system, provides a unique system for water splitting to molecular hydrogen and oxygen with solar to hydrogen efficiencies approaching 20%.
Demonstration of 28% solar to hydrogen efficiency and evaluation of its economic importance.
M.A. Khan, I. Al-Shankiti, A. Ziani, H. Idriss, Sustainable Energy and Fuel. 5, 1085-1094 (2021)
https://pubs.rsc.org/en/Content/ArticleLanding/2021/SE/D0SE01761B#!divAbstract
Towards large scale hydrogen production from water, what have we learned and what are the main hurdles to cross for commercialization.
H. Idriss, Energy Technology, 9, 2000843 (2021)
https://doi.org/10.1002/ente.202000843
A Stable Integrated Photoelectrochemical Reactor for H2 Production from Water Attains a Solar-to-Hydrogen Efficiency of 18% at 15 Suns and 13% at 207 Suns. M.A. Khan, I. Al-Shankiti, A. Ziani, N. Wehbe, H. Idriss , Angewandte Chemie, Int. Ed. 59, 14802–14808 (2020).
2. Fundamental studies of the surface reactions of CeO2
The potential of CeO2 as an epoxidation catalyst is studied for the reaction of propylene with H2O2 by Fourier transform infrared (FTIR) spectroscopy and temperature programmed desorption (TPD). It demonstrated room temperature activation of adsorbed hydrogen peroxide and gas phase propylene, producing adsorbed C3H6O most likely through Eley-Riedel (E-R) mechanism.
The reaction of hydrogen peroxide and propylene to propylene oxide on the surface of CeO2: An in situ FTIR spectroscopy and temperature programmed desorption study.
S. Bashir and H. Idriss J. Chem. Physics (Invited), 152, 044712 (2020).
https://doi.org/10.1063/1.5140544
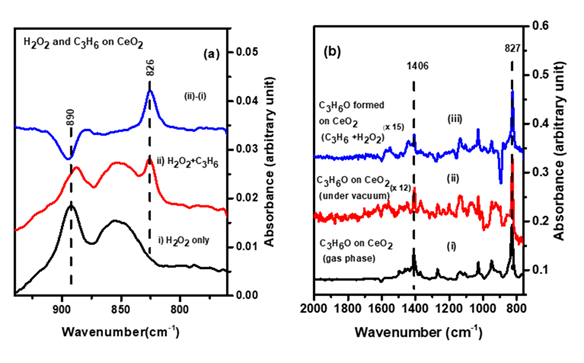
IR spectra of (a) propylene oxide during the reaction of propylene with adsorbed H2O2 on CeO2 (b) propylene oxide in the gas phase and under vacuum compared to that of the reaction product observed in (a)
3. Actinide oxides
Study of the surfaces of the oxides of actinides. Their unique orbital symmetry and non-negligible relativistic effect on their electronic configuration is poised to lead to specific structure-sensitivity relationships in redox chemical reactions not encountered in transition metal oxides. These when coupled with plasmonic resonance of metal clusters and particles provide a prototype to study electron transfer reactions between the U5f and Au5d bands, triggered by photonic and thermal energies.
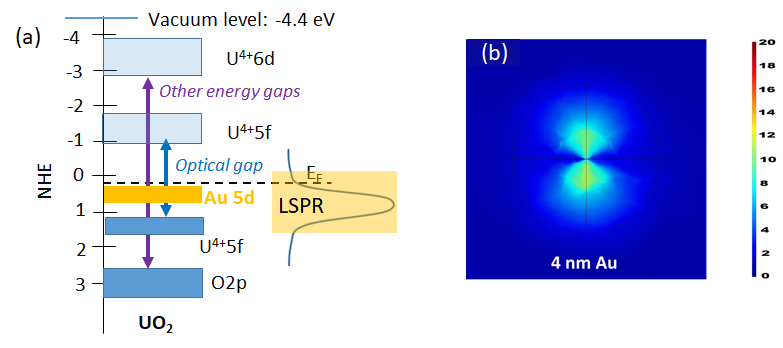
(a) Energy levels of the optical and electronic band gaps of UO2 superimposed on them the expected Au5d/6s orbital levels of a Au cluster. (b) Simulated Electric Field, EF (XY plane), at the interface of TiO2 on top of 4 nm Au thin film, the scale bar shows the EF intensity (from Khan et al. Catalysis Letters 147 (4) 811 (2017)
