Oxygen-sensitive microcavity arrays
O2-sensitive microcavity arrays
The basis of most of the work of the 3D cell culture systems group is the microcavity array system (fig. 1). The polymer foil based 3D cell culture system has been developed at the KIT and unites several advantages, such as their thin walls and therefore their high transparency which makes them idela for microscopic applications, the defined position of each cavity and more. The system has successfully been used for the (co-)culture of liver, heart, gut, several types of stem cells and other cell types and can also be used in specialized microbioreactors which allow for dynamic culture conditions.
Fig. 1: Sensorarrays for the culture of spheroids/organoids and simultaneous oxygen measurement in the microenvironment of the cells
Oxygen is the most important cell culture parameter which in most cell culture systems is not determined routinely or, like in 3D cultures, cannot be determined at all so far. By coating the array foil material with an oxygen-sensitive fluorophore, it is possible to measure oxygen label-free, in real-time, and without analyte consumption in the direct microenvironment of organoids and their surrounding. By this, the oxygen concentration or gradients in the direct microenvironment of hundreds of organoids can be measured simultaneously (see Fig. 2). The interesting feature of this new technology is the fact that cells can be analysed with regard to their oxygen consumption without subjecting them to hypoxia, making it possible to not only monitor the cells' beahviour prior to the assay (which is not possible with current systems) but moreover, to continue the cultivation after e.g. a mitochondrial stress test as long as needed.
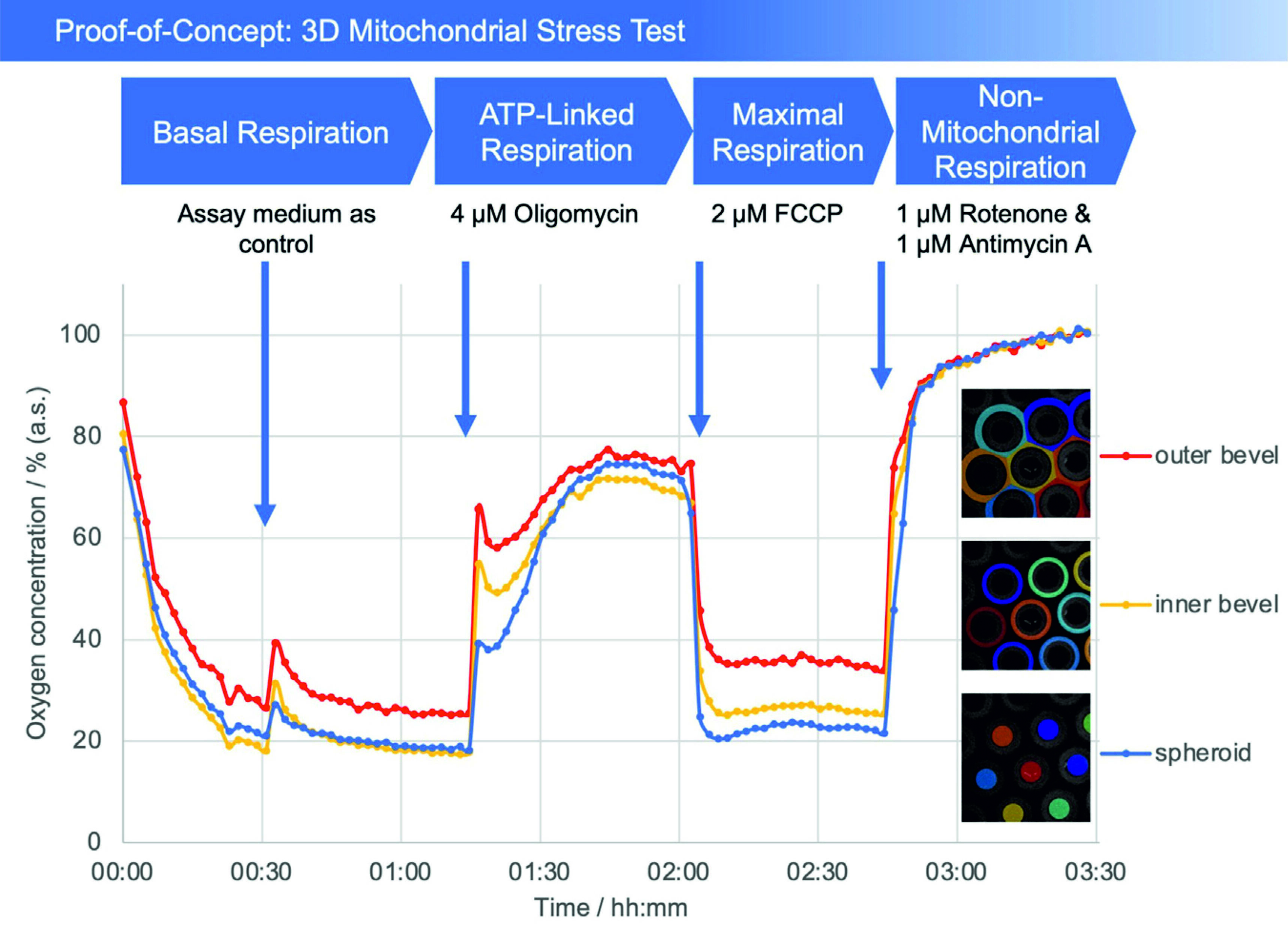
Fig. 2: Plot of oxygen concentration in the sensor array (average of the number of the detected microcavities) during the mitochondrial stress test. 4 μM oligomycin, 2 μM FCCP, and 1 μM of rotenone and antimycin A, respectively, were added. The oxygen values are measured with open cavities, no closed microcompartment is formed and oxygen is not consumed during the measurement process. The respective spheroid in its cavity generates an oxygen (post) diffusion equilibrium with the medium, thus is in a stress-free state under optimal cultivation conditions. Closed microcompartments, on the other hand, induce temporary hypoxic (stress) situations by cutting off oxygen post-diffusion (e.g., OCR measurements Seahorse/Agilent), even the measurement process itself can result in hypoxia when polarographic sensors (electrodes) are used. Methodically or sensorically induced hypoxia is avoided with the new method, furthermore hyperoxic situations can be detected by online monitoring and controlled, e.g., via incubator gassing changes. The overall outcome is that the spheroid can be cultured stress-free and physioxically, so that the mitochondrial stress caused by the test compounds can be optimally quantified.
