Chemical Biology of Precision Biomaterials Publications
The Department of Chemical Biology and Precision Biomaterials ranges from organ-targeted drug delivery to autonomous high-throughput biomaterials development for 3D tissue printing and organoids reconstruction in personalized medicine.
Selected Publications

Göckler, R.; Rösch, A.; Kirchner, S.; Elbuga-Ilica, R.; Seliwjorstow, A.; Fuhr, O.; Schepers, U.; Pianowski, Z. J Am Chem Soc. 2025, 147, 26652-26662
This study introduces a water-based synthesis of GelNB, which with GelS forms the fast-curing GelNB/GelS hydrogel. Compared to GelMA, it requires less photoinitiator, cures in seconds, and shows higher homogeneity and biocompatibility.
Read full article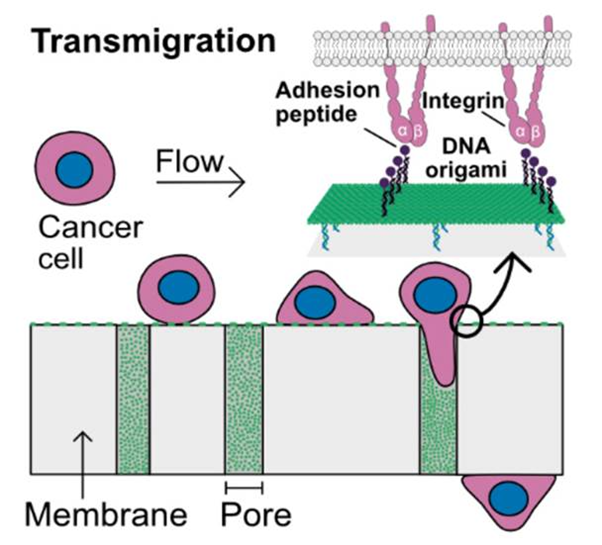
García-Chamé, M; Wadhwani, P.; Pfeifer, J.; Schepers, U.; Niemeyer, C. M.; Domínguez, C. M.; Angew Chem Int Ed Engl. 2024, 63, e202318805
The vasquip, a microfluidic bloss vessel system, which was developed by the Schepers group was treated with DNA origami nanostructures that mimics endothelial barriers to study cancer cell extravasation. The device simulates physiological flow conditions and allows direct visualization of cell transmigration through microchannel pores using 3D confocal imaging.
Read full article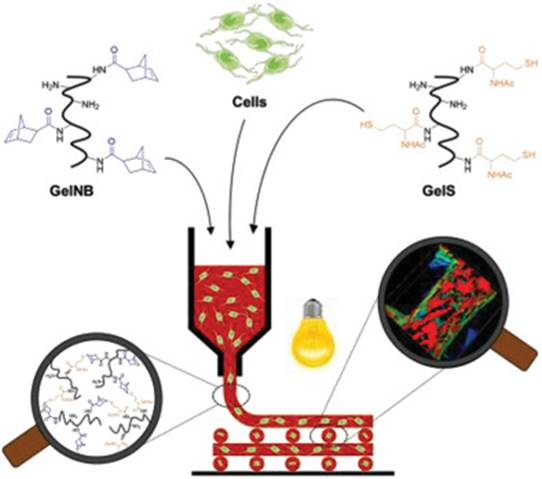
Göckler, T.; Haase, S.; Kempter, X.; Pfister, R.; Maciel, B. R.; Grimm, A.; Molitor, T.; Willenbacher, N.; Schepers, U. Adv Healthc Mater. 2021, 10, e2100206
This study introduces a water-based synthesis of GelNB, which with GelS forms the fast-curing GelNB/GelS hydrogel. Compared to GelMA, it requires less photoinitiator, cures in seconds, and shows higher homogeneity and biocompatibility.
Read full articleAll Publications
-
3D‐Printed Hydrogels from Recycled Cellulose for Biomedical Applications
Yousefshahi, S.; Pohl, E.; Sehn, T.; Jungbluth, M.; Huber, B.; Klein, C. O.; Beuermann, S.; Meier, M. A. R.; Schepers, U.; Schmitt, C. W.; Théato, P.
2026. ChemSusChem, 19 (1), 1. doi:10.1002/cssc.202501734
-
Photoswitchable Fluorescence of Peptide-Based Hemipiperazines Inside of Living Cells
Gödtel, P.; Rösch, A.; Kirchner, S.; Elbuga-Ilica, R.; Seliwjorstow, A.; Fuhr, O.; Schepers, U.; Pianowski, Z.
2025. Journal of the American Chemical Society, 147 (30), 26652–26662. doi:10.1021/jacs.5c07013
-
Modulating the photolysis of aryl azides in a supramolecular host to develop photoactivatable fluorophores
Qiu, X.; Pohl, E.; Jung, A.; Cai, Q.; Su, H.; Fuhr, O.; Schepers, U.; Bräse, S.
2024. Chemical Communications, 60 (88), 12856–12859. doi:10.1039/d4cc03907f -
A simple method to modulate the selectivity of aryl azide photolysis using cucurbit[8]uril
Qiu, X.; Cai, Q.; Pohl, E.; Jung, A.; Su, H.; Fuhr, O.; Schepers, U.; Bräse, S.
2024. Chemical Communications, 60 (88), 12852–12855. doi:10.1039/d4cc04209c -
A Versatile Microfluidic Platform for Extravasation Studies Based on DNA Origami—Cell Interactions
García-Chamé, M.; Wadhwani, P.; Pfeifer, J.; Schepers, U.; Niemeyer, C. M.; Domínguez, C. M.
2024. Angewandte Chemie International Edition, 63 (28), Art.-Nr.: e202318805. doi:10.1002/anie.202318805 -
Block Polyelectrolyte Additives That Modulate the Viscoelasticity and Enhance the Printability of Gelatin Inks at Physiological Temperatures
Göckler, T.; Albreiki, F.; Li, D.; Grimm, A.; Mecklenburg, F.; Urueña, J. M.; Schepers, U.; Srivastava, S.
2024. ACS Applied Polymer Materials, 6 (5), 2427–2441. doi:10.1021/acsapm.3c01085 -
Modulating and Accelerating Photolysis of Photoactivatable [2.2]Paracyclophane Aryl Azide in Supramolecular Host for Bioimaging
Qiu, X.; Pohl, E.; Cai, Q.; Seibert, J.; Li, Y.; Leopold, S.; Fuhr, O.; Meier, M. A. R.; Schepers, U.; Bräse, S.
2024. Advanced Functional Materials, Art.-Nr.: 2401938. doi:10.1002/adfm.202401938 -
Cocktail of lipophilic and hydrophilic chemotherapeutics in high-load core@shell nanocarriers to treat pancreatic tumours
Rudolph, D.; Ischyropoulou, M.; Pfeifer, J.; Napp, J.; Schepers, U.; Alves, F.; Feldmann, C.
2024. Nanoscale Advances, 6 (3), 973–984. doi:10.1039/D3NA00720K -
Azide Thermolysis Frameworks: Self‐inflating, Porous, and Lightweight Materials
Brückel, J.; Matt, Y.; Schmidt, L.; Mattern, C. M.; Schepers, U.; Leopold, S.; Calkovsky, M.; Gerthsen, D.; Bräse, S.
2024. ChemNanoMat, 10 (2), Art.-Nr.: e202300222. doi:10.1002/cnma.202300222 -
Modulating Aryl Azide Photolysis: Synthesis of a Room‐Temperature Phosphorescent Carboline in Cucurbit[7]uril Host
Qiu, X.; Wang, Y.; Leopold, S.; Lebedkin, S.; Schepers, U.; Kappes, M. M.; Biedermann, F.; Bräse, S.
2024. Small, 20 (16), Art.Nr.: 2307318. doi:10.1002/smll.202307318
-
Printed Electronic Devices and Systems for Interfacing with Single Cells up to Organoids
Saghafi, M. K.; Vasantham, S. K.; Hussain, N.; Mathew, G.; Colombo, F.; Schamberger, B.; Pohl, E.; Marques, G. C.; Breitung, B.; Tanaka, M.; Bastmeyer, M.; Selhuber-Unkel, C.; Schepers, U.; Hirtz, M.; Aghassi-Hagmann, J.
2023. Advanced Functional Materials, 33 (51), Art.-Nr.: 2308613. doi:10.1002/adfm.202308613 -
Targeted micro-heterogeneity in bioinks allows for 3D printing of complex constructs with improved resolution and cell viability
Maciel, B. R.; Grimm, A.; Oelschlaeger, C.; Schepers, U.; Willenbacher, N.
2023. Biofabrication, 15 (4), Art.-Nr.: 045013. doi:10.1088/1758-5090/acee22 -
Bioconjugation in Materials Science
Bednarek, C.; Schepers, U.; Thomas, F.; Bräse, S.
2023. Advanced Functional Materials, 34 (20), Art.-Nr.: 2303613. doi:10.1002/adfm.202303613 -
Theranostic inorganic–organic hybrid nanoparticles with a cocktail of chemotherapeutic and cytostatic drugs
Khorenko, M.; Pfeifer, J.; Napp, J.; Meschkov, A.; Alves, F.; Schepers, U.; Feldmann, C.
2023. Journal of Materials Chemistry B, 11 (16), 3635–3649. doi:10.1039/D3TB00226H
-
Polyelectrolyte Complex-Covalent Interpenetrating Polymer Network Hydrogels
Li, D.; Göckler, T.; Schepers, U.; Srivastava, S.
2022. Macromolecules, 55 (11), 4481–4491. doi:10.1021/acs.macromol.2c00590 -
Polyelectrolyte Complex-Covalent Interpenetrating Polymer Network Hydrogels
Li, D.; Göckler, T.; Schepers, U.; Srivastava, S.
2022. American Chemical Society (ACS). doi:10.26434/chemrxiv-2022-b73xh -
The jasmonate biosynthesis Gene OsOPR7 can mitigate salinity induced mitochondrial oxidative stress
Asfaw, K. G.; Liu, Q.; Eghbalian, R.; Purper, S.; Akaberi, S.; Dhakarey, R.; Münch, S. W.; Wehl, I.; Bräse, S.; Eiche, E.; Hause, B.; Bogeski, I.; Schepers, U.; Riemann, M.; Nick, P.
2022. Plant science, 316, Article no: 111156. doi:10.1016/j.plantsci.2021.111156 -
Novel tetrameric cell penetrating antimicrobial peptoids effective against mycobacteria and drug-resistant Staphylococcus aureus
Fleck, B. S.; Mukherjee, D.; Tram, N. D. T.; Ee, P. L. R.; Schepers, U.
2022. Frontiers in bioscience (Landmark edition), 27 (2), Art.-Nr.: 64. doi:10.31083/j.fbl2702064 -
Evaluation of a Novel Thiol–Norbornene-Functionalized Gelatin Hydrogel for Bioprinting of Mesenchymal Stem Cells
Burchak, V.; Koch, F.; Siebler, L.; Haase, S.; Horner, V. K.; Kempter, X.; Stark, G. B.; Schepers, U.; Grimm, A.; Zimmermann, S.; Koltay, P.; Strassburg, S.; Finkenzeller, G.; Simunovic, F.; Lampert, F.
2022. International Journal of Molecular Sciences, 23 (14), Artkl.Nr.: 7939. doi:10.3390/ijms23147939
-
Bio-instructive materials on-demand-combinatorial chemistry of peptoids, foldamers, and beyond
Herlan, C. N.; Feser, D.; Schepers, U.; Bräse, S.
2021. Chemical Communications, 57 (85), 11131–11152. doi:10.1039/d1cc04237h -
Designing Inherently Photodegradable Cell‐Adhesive Hydrogels for 3D Cell Culture
Rosenfeld, A.; Göckler, T.; Kuzina, M.; Reischl, M.; Schepers, U.; Levkin, P. A.
2021. Advanced healthcare materials, 10 (16), Art.Nr. 2100632. doi:10.1002/adhm.202100632 -
Cyclic Peptoid-Peptide Hybrids as Versatile Molecular Transporters
Herlan, C. N.; Meschkov, A.; Schepers, U.; Bräse, S.
2021. Frontiers in Chemistry, 9, Art. Nr.: 696957. doi:10.3389/fchem.2021.696957 -
Total synthesis of decarboxyaltenusin
Warmuth, L.; Weiß, A.; Reinhardt, M.; Meschkov, A.; Schepers, U.; Podlech, J.
2021. Beilstein Journal of Organic Chemistry, 17, 224–228. doi:10.3762/BJOC.17.22 -
Intriguing Heteroleptic Zn
Tabone, R.; Feser, D.; Lemma, E. D.; Schepers, U.; Bizzarri, C.
2021. Frontiers in Chemistry, 9, 754420. doi:10.3389/fchem.2021.754420 -
Tuning Superfast Curing Thiol-Norbornene-Functionalized Gelatin Hydrogels for 3D Bioprinting
Göckler, T.; Haase, S.; Kempter, X.; Pfister, R.; Maciel, B. R.; Grimm, A.; Molitor, T.; Willenbacher, N.; Schepers, U.
2021. Advanced Healthcare Materials, 10 (14), Art.-Nr. 2100206. doi:10.1002/adhm.202100206 -
Highly NIR-emitting ytterbium complexes containing 2-(tosylaminobenzylidene)-N-benzoylhydrazone anions: structure in solution and use for bioimaging
Kovalenko, A. D.; Pavlov, A. A.; Ustinovich, I. D.; Kalyakina, A. S.; Goloveshkin, A. S.; Marciniak, Ł.; Lepnev, L. S.; Burlov, A. S.; Schepers, U.; Bräse, S.; Utochnikova, V. V.
2021. Dalton Transactions, 50 (11), 3786–3791. doi:10.1039/d0dt03913f
-
A mitochondria-targeted coenzyme Q peptoid induces superoxide dismutase and alleviates salinity stress in plant cells
Asfaw, K. G.; Liu, Q.; Xu, X.; Manz, C.; Purper, S.; Eghbalian, R.; Münch, S. W.; Wehl, I.; Bräse, S.; Eiche, E.; Hause, B.; Bogeski, I.; Schepers, U.; Riemann, M.; Nick, P.
2020. Scientific reports, 10, Article number:11563. doi:10.1038/s41598-020-68491-4 -
The Staudinger Ligation
Bednarek, C.; Wehl, I.; Jung, N.; Schepers, U.; Bräse, S.
2020. Chemical reviews, 120 (10), 4301–4354. doi:10.1021/acs.chemrev.9b00665
-
Author Correction: A Peptoid Delivers CoQ-derivative to Plant Mitochondria via Endocytosis
Asfaw, K. G.; Liu, Q.; Maisch, J.; Münch, S. W.; Wehl, I.; Bräse, S.; Bogeski, I.; Schepers, U.; Nick, P.
2019. Scientific reports, 9 (1), Art.Nr.: 18832. doi:10.1038/s41598-019-54996-0 -
Anti-Tumor Activity of Doxorubicin-loaded Boehmite Nanocontainers
Seidl, C.; Simonato, S.; Zittel, E.; Schepers, U.; Feldmann, C.
2019. Zeitschrift für anorganische und allgemeine Chemie, 645 (24), 1372–1378. doi:10.1002/zaac.201900211 -
A Peptoid Delivers CoQ-derivative to Plant Mitochondria via Endocytosis
Asfaw, K. G.; Liu, Q.; Maisch, J.; Münch, S. W.; Wehl, I.; Bräse, S.; Bogeski, I.; Schepers, U.; Nick, P.
2019. Scientific reports, 9 (1), Art.Nr.: 9839. doi:10.1038/s41598-019-46182-z -
Synthesis, Characterization, and Biological Properties of Steroidal Ruthenium(II) and Iridium(III) Complexes Based on the Androst-16-en-3-ol Framework
Koch, V.; Meschkov, A.; Feuerstein, W.; Pfeifer, J.; Fuhr, O.; Nieger, M.; Schepers, U.; Bräse, S.
2019. Inorganic chemistry, 58 (23), 15917–15926. doi:10.1021/acs.inorgchem.9b02402 -
Zirconyl Hydrogenphosphate Nanocontainers for Flexible Transport and Release of Lipophilic Cytostatics, Insecticides, and Antibiotics
Rein, V.; Meschkov, A.; Hagens, K.; Redinger, N.; Schepers, U.; Mehlhorn, H.; Schaible, U. E.; Feldmann, C.
2019. Advanced functional materials, 29 (28), Art.Nr.: 1900543. doi:10.1002/adfm.201900543 -
New Polyfluorinated Cyanine Dyes for Selective NIR Staining of Mitochondria
Braun, A. B.; Wehl, I.; Kölmel, D. K.; Schepers, U.; Bräse, S.
2019. Chemistry - a European journal, 25 (34), 7998–8002. doi:10.1002/chem.201900412 -
On the design of new europium heteroaromatic carboxylates for OLED application
Koshelev, D. S.; Chikineva, T. Y.; Khudoleeva, V. Y.; Medvedko, A. V.; Vashchenko, A. A.; Goloveshkin, A. S.; Tsymbarenko, D. M.; Averin, A. A.; Meschkov, A.; Schepers, U.; Vatsadze, S. Z.; Utochnikova, V. V.
2019. Dyes and pigments, 170, 107604. doi:10.1016/j.dyepig.2019.107604 -
Controlling the Uptake of Diarylethene‐Based Cell‐Penetrating Peptides into Cells Using Light
Schober, T.; Wehl, I.; Afonin, S.; Babii, O.; Iampolska, A.; Schepers, U.; Komarov, I. V.; Ulrich, A. S.
2019. ChemPhotoChem, 3 (6), 384–391. doi:10.1002/cptc.201900019 -
3-D geometry and irregular connectivity dictate neuronal firing in frequency domain and synchronization
Ren, T.; Grosshäuser, B.; Sridhar, K.; Nieland, T. J. F.; Tocchio, A.; Schepers, U.; Demirci, U.
2019. Biomaterials, 197, 171–181. doi:10.1016/j.biomaterials.2019.01.017 -
Fluorescent Inorganic-Organic Hybrid Nanoparticles
Neumeier, B. L.; Khorenko, M.; Alves, F.; Goldmann, O.; Napp, J.; Schepers, U.; Reichardt, H. M.; Feldmann, C.
2019. ChemNanoMat, 5 (1), 24–45. doi:10.1002/cnma.201800310 -
Brightly luminescent lanthanide pyrazolecarboxylates: Synthesis, luminescent properties and influence of ligand isomerism
Utochnikova, V. V.; Abramovich, M. S.; Latipov, E. V.; Dalinger, A. I.; Goloveshkin, A. S.; Vashchenko, A. A.; Kalyakina, A. S.; Vatsadze, S. Z.; Schepers, U.; Bräse, S.; Kuzmina, N. P.
2019. Journal of luminescence, 205, 429–439. doi:10.1016/j.jlumin.2018.09.027
-
Lanthanide pyrazolecarboxylates for OLEDs and bioimaging
Utochnikova, V. V.; Latipov, E. V.; Dalinger, A. I.; Nelyubina, Y. V.; Vashchenko, A. A.; Hoffmann, M.; Kalyakina, A. S.; Vatsadze, S. Z.; Schepers, U.; Bräse, S.; Kuzmina, N. P.
2018. Journal of luminescence, 202, 38–46. doi:10.1016/j.jlumin.2018.05.022 -
Fluorescent Sulfonate-Based Inorganic–Organic Hybrid Nanoparticles for Staining and Imaging
Poß, M.; Zittel, E.; Meschkov, A.; Schepers, U.; Feldmann, C.
2018. Bioconjugate chemistry, 29 (8), 2818–2828. doi:10.1021/acs.bioconjchem.8b00423 -
Combinatorial Synthesis of Peptoid Arrays via Laser-Based Stacking of Multiple Polymer Nanolayers
Mattes, D. S.; Streit, B.; Bhandari, D. R.; Greifenstein, J.; Foertsch, T. C.; Münch, S. W.; Ridder, B.; v. Bojničić-Kninski, C.; Nesterov-Mueller, A.; Spengler, B.; Schepers, U.; Bräse, S.; Loeffler, F. F.; Breitling, F.
2018. Macromolecular rapid communications, 40 (6), Art.Nr.: 1800533. doi:10.1002/marc.201800533 -
Gd
Poß, M.; Zittel, E.; Seidl, C.; Meschkov, A.; Muñoz, L.; Schepers, U.; Feldmann, C.
2018. Advanced functional materials, 28 (32), 1801074. doi:10.1002/adfm.201801074 -
A new structure–activity relationship for cyanine dyes to improve photostability and fluorescence properties for live cell imaging
Schwechheimer, C.; Rönicke, F.; Schepers, U.; Wagenknecht, H.-A.
2018. Chemical science, 9, 6557–6563. doi:10.1039/C8SC01574K -
Click chemistry-mediated biotinylation reveals a function for the protease BACE1 in modulating the neuronal surface glycoproteome
Herber, J.; Njavro, J.; Feederle, R.; Schepers, U.; Müller, U.; Bräse, S.; Müller, S.; Lichtenthaler, S. F.
2018. Molecular & cellular proteomics, 17 (8), 1487–1501. doi:10.1074/mcp.RA118.000608 -
Remarkable high efficiency of red emitters using Eu(III) ternary complexes
Kalyakina, A. S.; Utochnikova, V. V.; Zimmer, M.; Dietrich, F.; Kaczmarek, A. M.; Van Deun, R.; Vashchenko, A. A.; Goloveshkin, A. S.; Nieger, M.; Gerhards, M.; Schepers, U.; Bräse, S.
2018. Chemical communications, 54 (41), 5221–5224. doi:10.1039/C8CC02930J -
“siRNA traffic lights”: arabino-configured 2′-anchors for fluorescent dyes are key for dual color readout in cell imaging
Steinmeyer, J.; Walter, H.-K.; Bichelberger, M. A.; Schneider, V.; Kubař, T.; Rönicke, F.; Olshausen, B.; Nienhaus, K.; Nienhaus, G. U.; Schepers, U.; Elstner, M.; Wagenknecht, H.-A.
2018. Organic & biomolecular chemistry, 16 (20), 3726–3731. doi:10.1039/C8OB00417J -
vasQchip : A Novel Microfluidic, Artificial Blood Vessel Scaffold for Vascularized 3D Tissues
Kappings, V.; Grün, C.; Ivannikov, D.; Hebeiss, I.; Kattge, S.; Wendland, I.; Rapp, B. E.; Hettel, M.; Deutschmann, O.; Schepers, U.
2018. Advanced Materials Technologies, 3 (4), Art.Nr. 1700246. doi:10.1002/admt.201700246 -
Durchblutete Organe auf dem Chip. Entwicklung eines vaskularisierten Organ-on-Chip System
Grün, C.; Pfister, R.; Haase, S.; Schepers, U.
2018. GIT, (2), 2–4 -
Reporting pH-sensitive drug release via unpaired spin fluorescence silencing
Eing, M.; Olshausen, B.; Fairfull-Smith, K. E.; Schepers, U.; Barner-Kowollik, C.; Blinco, J. P.
2018. Polymer chemistry, 9 (4), 499–505. doi:10.1039/C7PY01942D -
Fish-Microarray: A Miniaturized Platform for Single-Embryo High-Throughput Screenings
Popova, A. A.; Marcato, D.; Peravali, R.; Wehl, I.; Schepers, U.; Levkin, P. A.
2018. Advanced functional materials, 28 (3), 1703486. doi:10.1002/adfm.201703486
-
An optimized version of the Secretome Protein Enrichment with Click Sugars (SPECS) method leads to enhanced coverage of the secretome
Serdaroglu, A.; Müller, S. A.; Schepers, U.; Bräse, S.; Weichert, W.; Lichtenthaler, S. F.; Kuhn, P.-H.
2017. Proteomics, 17 (5), Art.Nr. 1600423. doi:10.1002/pmic.201600423 -
Lanthanide Fluorobenzoates as Bio-Probes : a Quest for the Optimal Ligand Fluorination Degree
Kalyakina, A. S.; Utochnikova, V. V.; Bushmarinov, I. S.; Le-Deygen, I. M.; Volz, D.; Weis, P.; Schepers, U.; Kuzmina, N. P.; Bräse, S.
2017. Chemistry - a European journal, 23 (59), 14944–14953. doi:10.1002/chem.201703543 -
Microemulsion-made gadolinium carbonate hollow nanospheres showing magnetothermal heating and drug release
Jung-König, J.; Sanhaji, M.; Popescu, R.; Seidl, C.; Zittel, E.; Schepers, U.; Gerthsen, D.; Hilger, I.; Feldmann, C.
2017. Nanoscale, 9 (24), 8362–8372. doi:10.1039/c7nr01784g -
Synthesis of Wavelength-Shifting Fluorescent DNA and RNA with Two Photostable Cyanine-Styryl Dyes as the Base Surrogate Pair
Steinmeyer, J.; Rönicke, F.; Schepers, U.; Wagenknecht, H.-A.
2017. ChemistryOpen, 6 (4), 514–518. doi:10.1002/open.201700059 -
Europium 2-benzofuranoate : Synthesis and use for bioimaging
Utochnikova, V. V.; Koshelev, D. S.; Medvedko, A. V.; Kalyakina, A. S. ..; Bushmarinov, I. S.; Grishko, A. Y.; Schepers, U.; Bräse, S.; Vatsadze, S. Z.
2017. Optical materials, 74, 191–196. doi:10.1016/j.optmat.2017.05.038 -
Surface modified EuₓLa₁₋ₓF₃ nanoparticles as luminescent biomarkers : Still plenty of room at the bottom
Khudoleeva, V.; Utochnikova, V. V.; Kalyakina, A. S.; Deygen, I. M.; Shiryaev, A. A.; Marciniak, Ł.; Lebedev, V. A.; Roslyakov, I. V.; Garshev, A. V.; Lepnev, L. S.; Schepers, U.; Bräse, S.; Kuzmina, N. P.
2017. Dyes and pigments, 143, 348–355. doi:10.1016/j.dyepig.2017.04.058 -
Double-Strand DNA Breaks Induced by Paracyclophane Gold(I) Complexes
Bestgen, S.; Seidl, C.; Wiesner, T.; Zimmer, A.; Falk, M.; Köberle, B.; Austeri, M.; Paradies, J.; Bräse, S.; Schepers, U.; Roesky, P. W.
2017. Chemistry - a European journal, 23 (26), 6315–6322. doi:10.1002/chem.201605237 -
A postsynthetically 2’-“clickable” uridine with arabino configuration and its application for fluorescent labeling and imaging of DNA
Walter, H.-K.; Olshausen, B.; Schepers, U.; Wagenknecht, H.-A.
2017. Beilstein journal of organic chemistry, 13, 127–137. doi:10.3762/bjoc.13.16
-
Novel Prodrug of Doxorubicin Modified by Stearoylspermine Encapsulated into PEG-Chitosan-Stabilized Liposomes
Deygen, I. M.; Seidl, C.; Kölmel, D. K.; Bednarek, C.; Heissler, S.; Kudryashova, E. V.; Bräse, S.; Schepers, U.
2016. Langmuir, 32 (42), 10861–10869. doi:10.1021/acs.langmuir.6b01023 -
A Modular Class of Fluorescent Difluoroboranes: Synthesis, Structure, Optical Properties, Theoretical Calculations and Applications for Biological Imaging
Bachollet, S. P. J. T.; Volz, D.; Fiser, B.; Münch, S.; Rönicke, F.; Carrillo, J.; Adams, H.; Schepers, U.; Gómez-Bengoa, E.; Bräse, S.; Harrity, J. P. A.
2016. Chemistry - a European journal, 22 (35), 12430–12438. doi:10.1002/chem.201601915 -
Chemical Synthesis of Glycosaminoglycans
Mende, M.; Bednarek, C.; Wawryszyn, M.; Sauter, P.; Biskup, M. B.; Schepers, U.; Bräse, S.
2016. Chemical reviews, 116 (14), 8193–8255. doi:10.1021/acs.chemrev.6b00010 -
Systematic substrate identification indicates a central role for the metalloprotease ADAM10 in axon targeting and synapse function
Kuhn, P.-H.; Colombo, A. V.; Schusser, B.; Dreymueller, D.; Wetzel, S.; Schepers, U.; Herber, J.; Ludwig, A.; Kremmer, E.; Montag, D.; Müller, U.; Schweizer, M.; Saftig, P.; Bräses, S.; Lichtenthaler, S. F.
2016. eLife, 5 (JANUARY2016), e12748. doi:10.7554/eLife.12748 -
Tin Tungstate Nanoparticles: A Photosensitizer for Photodynamic Tumor Therapy
Seidl, C.; Ungelenk, J.; Zittel, E.; Bergfeldt, T.; Sleeman, J. P.; Schepers, U.; Feldmann, C.
2016. ACS nano, 10 (3), 3149–3157. doi:10.1021/acsnano.5b03060 -
Leukocyte responses to immobilized patterns of CXCL8
Girrbach, M.; Rink, I.; Ladnorg, T.; Azucena, C.; Heissler, S.; Haraszti, T.; Schepers, U.; Schmitz, K.
2016. Colloids and surfaces / B, 142, 385–391. doi:10.1016/j.colsurfb.2016.03.004 -
Polarity Sensitive Bioorthogonally Applicable Far-Red Emitting Labels for Postsynthetic Nucleic Acid Labeling by Copper-Catalyzed and Copper-Free Cycloaddition
Eördögh, A.; Steinmeyer, J.; Peewasan, K.; Kele, P.; Schepers, U.; Wagenknecht, H.-A.
2016. Bioconjugate Chemistry, 27 (2), 457–464. doi:10.1021/acs.bioconjchem.5b00557
-
Bioprinting: Organe aus dem Drucker?
Schepers, U.
2015. Nachrichten aus der Chemie, 63 (3), 309–314. doi:10.1002/nadc.201590093 -
Gallic acid induces mitotic catastrophe and inhibits centrosomal clustering in HeLa cells
Tan, S.; Guan, X.; Grün, C.; Zhou, Z.; Schepers, U.; Nick, P.
2015. Toxicology in vitro, 30 (1, Part B), 506–513. doi:10.1016/j.tiv.2015.09.011 -
Highly luminescent, water-soluble lanthanide fluorobenzonates : Syntheses, structures and photophysics, Part I : Lanthanide pentafluorobenzoates
Kalyakina, A. S.; Utochnikova, V. V.; Bushmarinov, I. S.; Ananyev, I. V.; Eremenko, I. L.; Volz, D.; Rönicke, F.; Schepers, U.; Van Deun, R.; Trigub, A. L.; Zubavichus, Y. V.; Kuzmina, N. P.; Bräse, S.
2015. Chemistry - a European journal, 21 (49), 17921–17932. doi:10.1002/chem.201501816 -
Synthesis of new diketopiperazines, thiolation to thiodiketopiperazines, and examination of ateir ROS-generating properties
Zhong, S.; Wandler, A. E. E.; Schepers, U.; Nieger, M.; Bräse, S.
2015. European Journal of Organic Chemistry, (31), 6858–6871. doi:10.1002/ejoc.201500900 -
Secretome analysis identifies novel signal peptide peptidase-like 3 (Sppl3) substrates and reveals a role of Sppl3 in multiple golgi glycosylation pathways
Kuhn, P. H.; Voss, M.; Haug-Kröper, M.; Schröder, B.; Schepers, U.; Bräse, S.; Haass, C.; Lichtenthaler, S. F.; Fluhrer, R.
2015. Molecular & cellular proteomics, 14 (6), 1584–1598. doi:10.1074/mcp.M115.048298 -
Photophysical properties and synthesis of new dye-cyclooctyne conjugates for multicolor and advanced microscopy
Hörner, A.; Hagendorn, T.; Schepers, U.; Bräse, S.
2015. Bioconjugate chemistry, 26 (4), 718–724. doi:10.1021/acs.bioconjchem.5b00059 -
Functionalized triazolopeptoids-a novel class for mitochondrial targeted delivery
Althuon, D.; Rönicke, F.; Fürniss, D.; Quan, J.; Wellhöfer, I.; Jung, N.; Schepers, U.; Bräse, S.
2015. Organic & biomolecular chemistry, 13, 4226–4230. doi:10.1039/C5OB00250H
-
Bright Coppertunities: Multinuclear CuI Complexes with N-P Ligands and Their Applications
Wallesch, M.; Volz, D.; Zink, D. M.; Schepers, U.; Nieger, M.; Baumann, T.; Bräse, S.
2014. Chemistry - a European journal, 20 (22), 6578–6590. doi:10.1002/chem.201402060 -
Switchable fluorescence by click reaction of a novel azidocarbazole dye
Hörner, A.; Volz, D.; Hagendorn, T.; Furniss, D.; Greb, L.; Ronicke, F.; Nieger, M.; Schepers, U.; Bräse, S.
2014. RSC Advances, 4 (23), 11528–11534. doi:10.1039/C3RA47964A -
In vitro fluorescence and phototoxicity of β-SnWO₄ nanoparticles
Ungelenk, J.; Seidl, C.; Zittel, E.; Roming, S.; Schepers, U.; Feldmann, C.
2014. Chemical communications, 50, 6600–6603. doi:10.1039/C4CC00308J -
Cytotoxicity and NMR studies of platinum complexes with cyclooctadiene ligands
Enders, M.; Görling, B.; Braun, A. B.; Seltenreich, J. E.; Reichenbach, L. F.; Rissanen, K.; Nieger, M.; Luy, B.; Schepers, U.; Bräse, S.
2014. Organometallics, 33, 4027–4034. doi:10.1021/om500540x -
Cell-penetrating peptoids: introduction of novel cationic side chains
Kölmel, D. K.; Hörner, A.; Rönicke, F.; Nieger, M.; Schepers, U.; Bräse, S.
2014. European journal of medicinal chemistry, 79, 231–243. doi:10.1016/j.ejmech.2014.03.078
-
Luminescent cell-penetrating pentadecanuclear lanthanide clusters
Thielemann, D. T.; Wagner, A. T.; Rösch, E.; Kölmel, D. K.; Heck, J. G.; Rudat, B.; Neumaier, M.; Feldmann, C.; Schepers, U.; Bräse, S.; Roesky, P. W.
2013. Journal of the American Chemical Society, 135 (20), 7454–7457. doi:10.1021/ja403539t -
Amphiphilic peptoid transporters - synthesis and evaluation
Vollrath, S. B. L.; Fürniss, D.; Schepers, U.; Bräse, S.
2013. Organic and Biomolecular Chemistry, 11, 8197–8201. doi:10.1039/C3OB41139G -
Peptoid-based rare-earth (group 3 and lanthanide) transporters
Kölmel, D. K.; Rudat, B.; Schepers, U.; Bräse, S.
2013. European Journal of Organic Chemistry, (14), 2761–2765. doi:10.1002/ejoc.201300219 -
Rhodamine F: a novel class of fluorous ponytailed dyes for bioconjugation
Kölmel, D. K.; Rudat, B.; Braun, D. M.; Bednarek, C.; Schepers, U.; Bräse, S.
2013. Organic and Biomolecular Chemistry, 11, 3954–3962. doi:10.1039/C3OB40267C -
Peptoids and polyamines going sweet: Modular synthesis of glycosylated peotoids and polyamines using click chemistry
Fürniss, D.; Mack, T.; Hahn, F.; Vollrath, S. B. L.; Koroniak, K.; Schepers, U.; Bräse, S.
2013. Beilstein Journal of Organic Chemistry, 9, 56–63. doi:10.3762/bjoc.9.7 -
Structural Characterization of a Peptoid with Lysine-like Side Chains and Biological Activity using NMR and Computational Methods
Sternberg, U.; Birtalan, E.; Jakovkin, I.; Luy, B.; Schepers, U.; Bräse, S.; Muhle-Goll, C.
2013. Organic and Biomolecular Chemistry, 11 (4), 640–647. doi:10.1039/c2ob27039k
-
Cell Penetrating Peptoids (CPPos): Synthesis of a Small Combinatorial Library by Using IRORI MiniKans
Kölmel, D. K.; Fürniss, D.; Susanto, S.; Lauer, A.; Grabher, C.; Bräse, S.; Schepers, U.
2012. Pharmaceuticals, 5 (12), 1265–1281. doi:10.3390/ph5121265 -
Synthesis of Functionalized Glutamine- and Asparagine-Type Peptoids : Scope and Limitations
Cardenal, C.; Vollrath, S. B. L.; Schepers, U.; Bräse, S.
2012. Helvetica chimica acta, 95 (11), 2237–2248. doi:10.1002/hlca.201200451 -
Killing double bonds softly: the reduction of polymer-bound alkenes
Fürniss, D.; Schepers, U.; Bräse, S.
2012. RSC Advances, 2 (30), 11273–11278. doi:10.1039/c2ra22189f -
The plakotenins: biomimetic Diels-Alder reactions, total synthesis, structural investigations, and chemical biology
Bourcet, E.; Kaufmann, L.; Arzt, S.; Bihlmeier, A.; Klopper, W.; Schepers, U.; Bräse, S.
2012. Chemistry - a European journal, 18 (47), 15004–15020. doi:10.1002/chem.201201585 -
Secretome Protein Enrichment with Click Sugars Identifies Physiological Substrates of the Alzheimer Protease BACE1 in Primary Neurons
Kuhn, P. H.; Koroniak, K.; Hogl, S.; Colombo, A.; Zeitschel, U.; Willem, M.; Volbracht, C.; Schepers, U.; Imhof, A.; Hoffmeister, A.; Haass, C.; Roßner, S.; Bräse, S.; Lichtenthaler, S. F.
2012. The EMBO journal, 31 (14), 3157–3168. doi:10.1038/emboj.2012.173 -
Interkingdom Signaling: Integration, Conformation, and Orientation of N-Acyl-L-Homoserine Lactones in Supported Lipid Bilayers
Barth, C.; Jakubczyk, D.; Kubas, A.; Anastassacos, F.; Brenner-Weiss, G.; Fink, K.; Schepers, U.; Bräse, S.; Koelsch, P.
2012. Langmuir, 28 (22), 8456–8462. doi:10.1021/la301241s -
Deuterium labelled N-acyl-L-homoserine lactones (AHLs) - Inter-kingdom signalling molecules - synthesis, structural studies, interaction with model lipid membranes
Jakubczyk, D.; Barth, C.; Kubas, A.; Anastassacos, F.; Koelsch, P.; Fink, K.; Schepers, U.; Brenner-Weiß, G.; Bräse, S.
2012. Analytical and bioanalytical chemistry, 403 (2), 473–482. doi:10.1007/s00216-012-5839-4 -
Novel three-dimensional Boyden chamber system for studying transendothelial transport
Hebeiss, I.; Truckenmüller, R.; Giselbrecht, S.; Schepers, U.
2012. Lab on a Chip, 12, 829–834. doi:10.1039/c2lc20733h
-
Photophysical properties of fluorescently-labeled peptoids
Rudat, B.; Birtalan, E.; Vollrath, S. B. L.; Fritz, D.; Kölmel, D. K.; Nieger, M.; Schepers, U.; Müllen, K.; Eisler, H.-J.; Lemmer, U.; Bräse, S.
2011. European Journal of Medicinal Chemistry, 46 (9), 4457–4465. doi:10.1016/j.ejmech.2011.07.020 -
Bioconjugation via azide-Staudinger ligation: an overview
Schilling, C. I.; Jung, N.; Biskup, M. B.; Schepers, U.; Bräse, S.
2011. Chemical Society Reviews, 40 (9), 4840–4871. doi:10.1039/C0CS00123F -
Investigating rhodamine B-labelled peptoids: Scopes and Limitations of its applications
Birtalan, E.; Rudat, B.; Kölmel, D. K.; Fritz, D.; Vollrath, S. B. L.; Schepers, U.; Bräse, S.
2011. Peptide science, 96 (5), 694–701. doi:10.1002/bip.21617 -
Goldnanopartikel als Radiosensitizer in menschlichen Tumorzellen bei der Bestrahlung mit 50 kV und 6 MV Photonen
Burger, N.; Veldwijk, M. R.; Bednarek, C.; Schepers, U.; Herskind, C.; Wenz, F.
2011. Strahlentherapie und Onkologie / Supplement, 17. Jahreskongress der Deutschen Gesellschaft für Radioonkologie (DEGRO 2011), Wiesbaden, 2.-5.Juni 2011, (Abstract P189), 124 (1), 124
-
Modified GSMP synthesis greatly improves the disulfide crosslink of T7 run-off siRNAs with cell penetrating peptides
Schmitz, K.; Hahn, F.; Schepers, U.
2010. Synlett, 2010, 2959–63. doi:10.1055/s-0030-1259022
-
Thieme chemistry journal awardees - where are they now? A convenient route for introduction of lipophilic side chains in polyamine backbones by solid-phase synthesis
Hahn, F.; Schepers, U.
2009. Synlett, (2009), (17), 2755–60. doi:10.1055/s-0029-1217987
-
Conjugation of spermine facilitates cellular uptake and enhances antitumor and antibiotic properties of highly lipophilic porphyrins
Hahn, F.; Schmitz, K.; Balaban, T. S.; Bräse, S.; Schepers, U.
2008. ChemMedChem, 3, 1185–88. doi:10.1002/cmdc.200800013
-
Solid-phase synthesis, bioconjugation, and toxicology of novel cationic oligopeptoids for cellular drug delivery
Schröder, T.; Schmitz, K.; Niemeier, N.; Balaban, T. S.; Krug, H. F.; Schepers, U.; Bräse, S.
2007. Bioconjugate Chemistry, 18, 342–54. doi:10.1021/bc0602073
